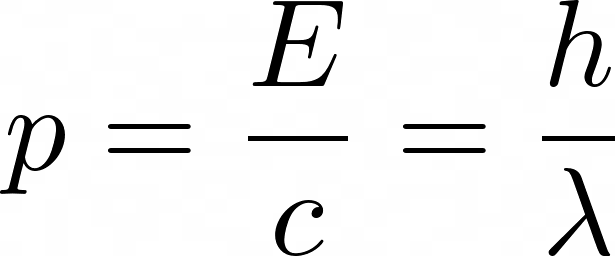De Broglie and the Wave-Particle Duality
Welcome!
Hey everyone! Welcome to my blog.
In here, I will be covering all sorts of things, from the obvious STEM-related content I’ve already been putting out on my pages to other things that just run through my mind. Today, in commemoration of de Broglie’s birthday, we will talk a bit about his groundbreaking contributions to quantum theory.
Some History
Let’s first talk about de Broglie himself:
Louis de Broglie (1892-1987)
De Broglie was a French physicist whose contributions to the early theory of quantum mechanics are immeasurable. His 1924 PhD thesis gave us the de Broglie hypothesis, which predicted that electrons wave properties. De Broglie won the Nobel Prize for Physics in 1929 after the wave-like behaviour of matter was experimentally demonstrated in 1927.
Now, a bit about light. Through experiments like Young’s double slit experiment and the theoretical frameworks set by Maxwell and his peers, light was thought to consist of electromagnetic waves while matter was thought to consist of particles. However, this resulted in some issues. In studying blackbody radiation, which is the electromagnetic radiation all objects emit, there was a bit of a theoretical malfunction. Under the wave theory of light, it seemed as if the energy these objects emitted was infinite, which made no sense!
Classical theory (shown by the black line) could not accurately predict experimental observation and gave illogical conclusions
More on this, and the technicalities behind the math, can be found in my book Advanced Calculus Explored. This wave notion of light then came under strict scrutiny when physicist Max Planck suggested that light is emitted in discrete ‘quanta’ of energy, a proposal that actually ended up resolving the blackbody radiation dilemma. This particle theory of light was further solidified by Einstein’s introduction of the photoelectric effect.
A Simple Derivation
These ‘quanta,’ now called photons, would have an energy defined by the Planck-Einstein relationship:
Here, E is energy, v is frequency, and h is the Planck constant. Einstein also postulated that photons have a momentum equal to their energy divided by the speed of light, c, i.e.
Here, λ (the Greek letter Lambda), represents the wavelength of the photon. De Broglie, in a very daring move, said why not extend this to matter too! He rearranged the above equation and came up with the well-known de Broglie Wavelength. De Broglie suggested this would be the characteristic wavelength for electrons.
What Does This Mean?
Although originally only meant for electrons, the relationship is now known to hold for all types of matter. Yes, all matter exhibits properties of both particles and waves — and matter’s characteristic “wavelength” can be found through de Broglie’s relation.
Now, what does this mean? Well, scientists cannot agree. Some theories treat either the particle or the wave aspect as an object’s fundamental nature, and seek to explain the other as an emergent property. The most accepted interpretation of quantum mechanics, the Copenhagen interpretation, dismisses the question about the underlying reality behind this duality. This is part of the much bigger debate around the wave-particle duality of quantum objects.
Did you enjoy reading this? If so, make sure to subscribe to my blog so you get updates. I will be posting a new blog post hopefully every week!